Which Best Describes an Aqueous Solution of Copper Chloride
The addition of 12M sulfuric acid reverses the changes through the copper. Aqueous copper sulphate solution blue in colour gives.
The class of this reaction is.
. Then write the ionic equation showing all. The chemical equation given to you is actually incorrect because copperII chloride CuCl_2 is not insoluble in aqueous solution. 1 pts Question 29 Which diagram best describes an aqueous solution of potassium chloride KCH.
Which of the following best describes what is in an aqueous solution of silver nitrate AgNO3aq. A solution of silver nitrate is mixed with a solution of sodium chloride resulting in a precipitate of silver chloride and a solution of sodium nitrate. The results obtained showed that these two adsorbents were effective for the adsorption of copper II ions in an aqueous solution.
A dark coating of copper metal appears on the zinc within two minutes and when 45 minutes have elapsed there is a thick coat of copper metal powder on the zinc strip and the blue color of the solution has lightened. Which of these pairs of substances in aqueous solution would constitute a buffer. Previous question Next question.
The adsorption kinetics obtained was that of the pseudo second-order for our two adsorbents. When zinc metal is immersed in a solution of 01 M aqueous copperII sulfate solution c opper metal plates out on the zincThe solution is initially blue in color. A concentrated copperII chloride solution B concentrated hydrochloric acid C.
Water molecules are omitted for clarity. Question 5 of 32 Scientists are trying to understand the composition of substances on different planets. View the full answer.
In these solutions copper I chloride shows a behaviour similar to that observed in pure CuCl NaCl HCl solutions. It is blue in colour due to the presence of. Write a balanced equation for the reaction that occurs when an aqueous solution of ironII chloride is mixed with an aqueous solution o potassium hydroxide.
HCl KOH. C Copper turnings precipitate zinc. Aqueous solution of X power supply Hydrogen is produced at the cathode and chlorine is produced at the anode.
With a view to industrial applications solubilities densities and electrical conductivi- ties of aqueous copper I and copper II. 100 3 ratings Electrolytes are substances that can dissociate into. When concentrated ammonia solution ammonium hydroxide is added to a clear light blue aqueous solution of copper II chloride a powdery light blue precipitate of copper II hydroxide forms.
HCl KCl b. Plan the problem. What volume of an 800 M solution of NaOH would be needed to precipitate all copper ions from 250.
We review their content and use your feedback to keep the quality high. Copper II chloride is a mild oxidant. Which diagram best describes an aqueous solution of potassium chloride KCI.
A concentrated aqueous copperII chloride solution is bright green in A concentrated aqueous copperII chloride solution is bright green in color. Further addition of ammonia causes the copper ion to go back into solution as a deep blue ammonia complex. With a view to industrial applications solubilities densities and electrical conductivities of aqueous copper I and copper II chlorides were measured in solutions containing other chlorides like those of iron zinc sodium and hydrogen.
Upon heating a sample of NiCl26H2O a sample originally weighing 35 g yields an anhydrous salt residue weighing 21g. A 4590 g sample of pure copper is heated in a test tube to 9940C. Students also viewed these Organic Chemistry questions Concentrated aqueous ammonia contains 100 mol NH3 dissolved in 244 mol H2O.
What is the net ionic equation that occurs when sodium hydroxide is added to an aqueous solution of formic. 9 The diagram shows the electrolysis of an aqueous solution of X using inert electrodes. Copper Chloride can irritate the stomach causing salivation nausea vomiting stomach pain and diarrhea.
When aqueous solutions of copperII chloride and potassium phosphate are mixed a precipitate of copperII phosphate is formed. Which best describes how scientists are proba. Contact can severely irritate and burn the skin and eyes with possible eye damage.
2 CuCl2 2 CuCl Cl. When diluted with water the solution becomes light blue. B Aqueous ammonia gives a white precipitate soluble in excess of the reagent.
Which of the following best describes a solution of sodium sulfate in water A A from CHEM 1211L at University Of Georgia. 6 Which statement describes the structure of an. Water molecules are omitted for clarity.
Copper chloride exists in a plethora of crystal structures and a variety of species in aqueous solution22it is an important species in hydrothermal fluids and several experimental studies have investigated copper chloride chemistry to better understand metal solubility transport and ore deposition mechanisms in geological. View the full answer. Explain these experimental results.
Aqueous sodium chloride NaCl will not react with aqueous copperII sulfate CuSO_4 because the two potential products are soluble in aqueous solution. Immediately or shortly after exposure to Copper Chloride. D Sodium hydroxide solution gives a white precipitate insoluble in excess of the reagent.
I a green precipitate with aqueous potassium fluoride and ii a bright green solution with aqueous potassium chloride. Solubilities densities and electrical conductivities of aqueous copper I and copper H chlorides in solutions containing other chlorides such as iron zinc sodium and hydrogen chlorides. Which diagram best describes an aqueous solution of potassium chlorideKCl.
The Langmuir isotherm was the one that best described the adsorption process for the two adsorbents. Breathing Copper Chloride can irritate the nose throat and lungs causing coughing and wheezing. It decomposes to copper I chloride and chlorine gas near 1000 C.
Water molecules are omitted for clarity. 5 What correctly describes the molecules in very dilute sugar solution at room temperature. The correct answer is option b as KCl is a strong electr.
A KC B INO Je OD. The reaction of 50 mol of Fe CO5 80 mol of PF3 and 60 mol of H2 will release ________ mol of CO. Water molecules are omitted for clarity Datasheet and Periodic Table K a ci K ar C КО KI ka K 02.
Write and balance the molecular equation first making sure that all formulas are correct. Solution for Which diagram best describes an aqueous solution of potassium chlorideKCl. Aqueous CuSO 4 exists as CuH 2 O 4SO 4.
Bly working on this problem. Copper II chloride CuCl2 reacts with several metals to produce copper metal or copper I chloride CuCl with oxidation of the other metal. What is the molar mass of anhydrous nickelII chloride hexahydrate NiCl26H2O.
In fact it is quite soluble. Sugar molecules water molecules. KHPO4 K2HPO4 c.
This means that the reaction does not produce. KOH KCl d. Scientists with the same perspectives use similar experiments so that they can compare results and draw the same conclusions.
ML of a 4. Write a balanced net ionic equation for this reaction.
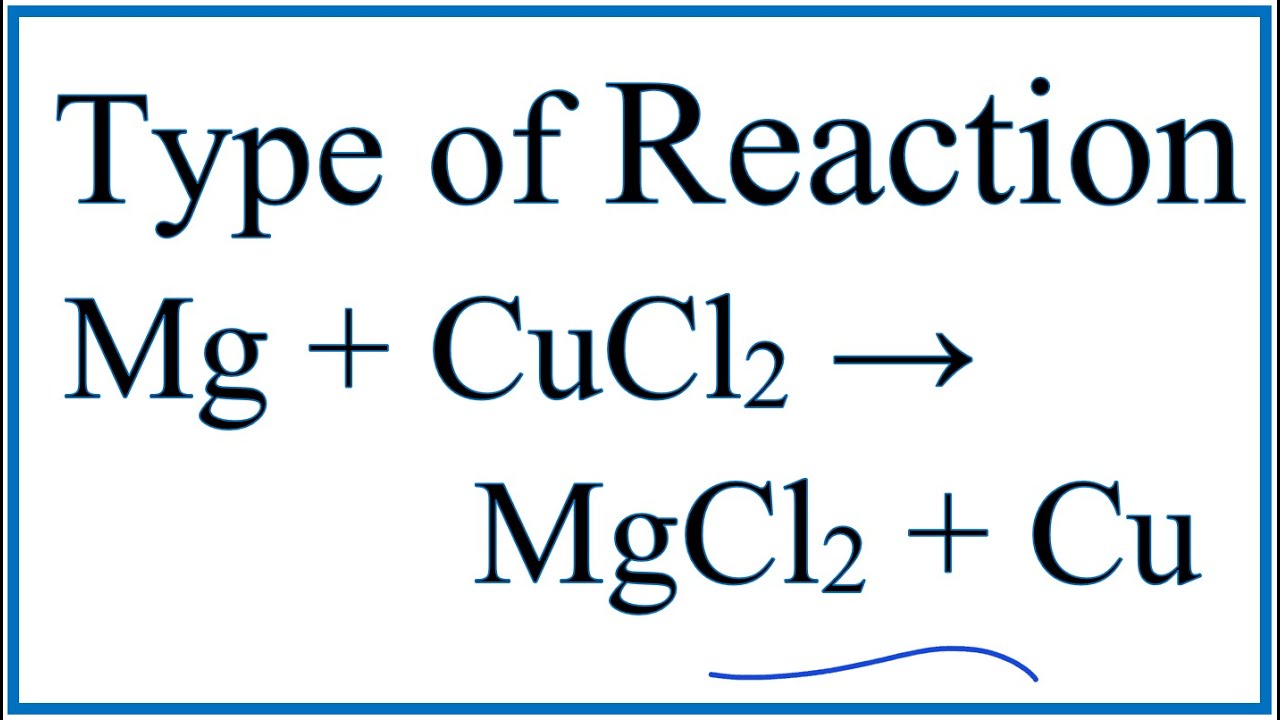
Type Of Reaction For Mg Cucl2 Mgcl2 Cu Youtube

Specific Absorption Coefficient Spectra Of Copper Ii Chloride Download Scientific Diagram

What Type Of Bond Is Copper Chloride Quora

How To Write The Net Ionic Equation For Cucl2 Na3po4 Cu3 Po4 2 Nacl Youtube

What Type Of Bond Is Copper Chloride Quora

Illustrative Potential Ph Diagram For 70 30 Brass In 0 1m Chloride Download Scientific Diagram
What Happens When You Mix Sodium Chloride And Copper Sulfate Quora

Solved A Creating Copper 11 Hydroxide Equilibrium Mixtures Chegg Com
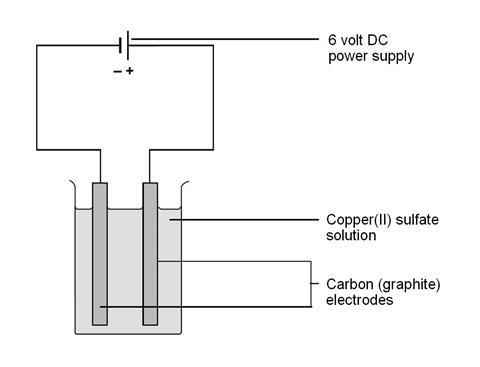
Electrolysis Of Copper Ii Sulfate Solution Experiment Rsc Education
Why Are Cobalt Chloride And Copper Sulfate Able To Indicate The Presence Of Water But Other Chemicals Such As Copper Chloride Are Not Able To Quora
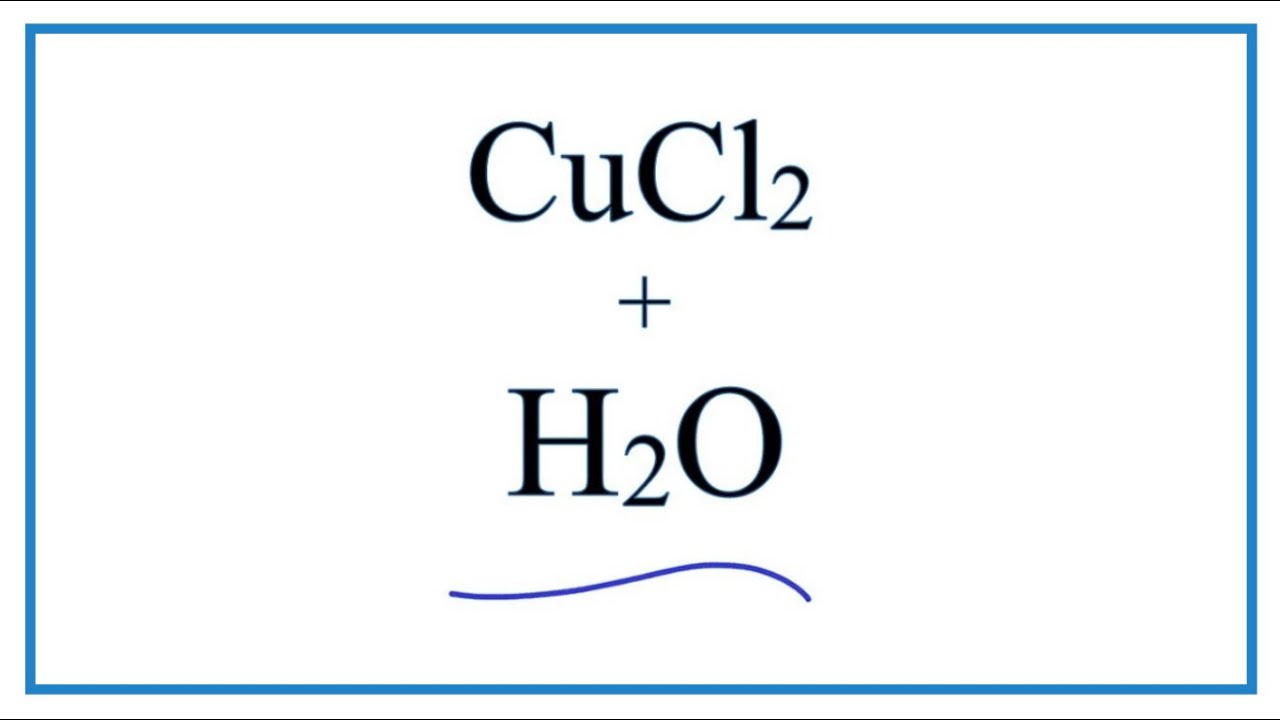
Equation For Cucl2 H2o Copper Ii Chloride Water Youtube

Is Copper Chloride With Water A Physical Reaction Quora

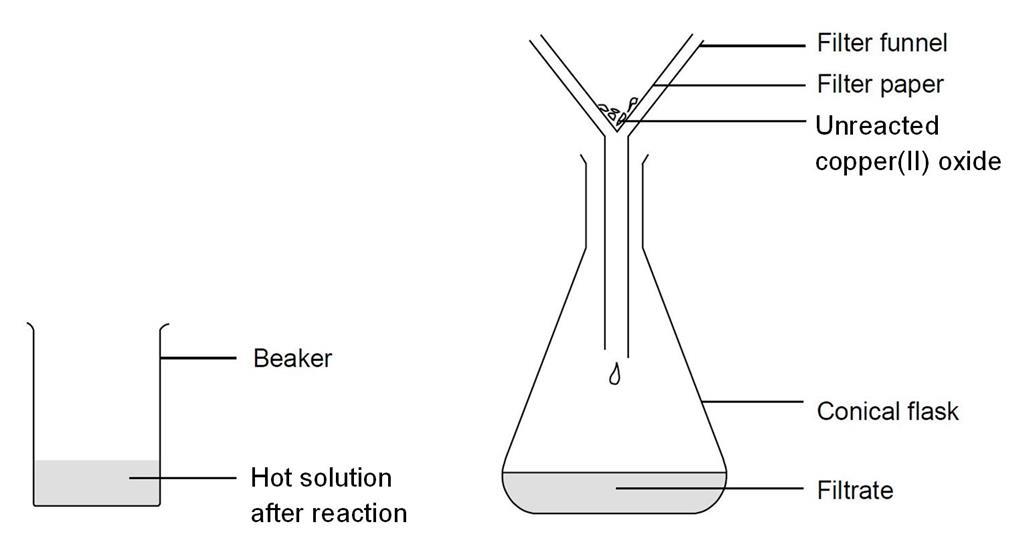
Reacting Copper Ii Oxide With Sulfuric Acid Experiment Rsc Education

Single Displacement Reaction Zinc And Copper Ii Ion Redox Chemdemos

Chemical Garden Periodic Table Of Videos Cool Experiments Science Activities For Kids Chemical
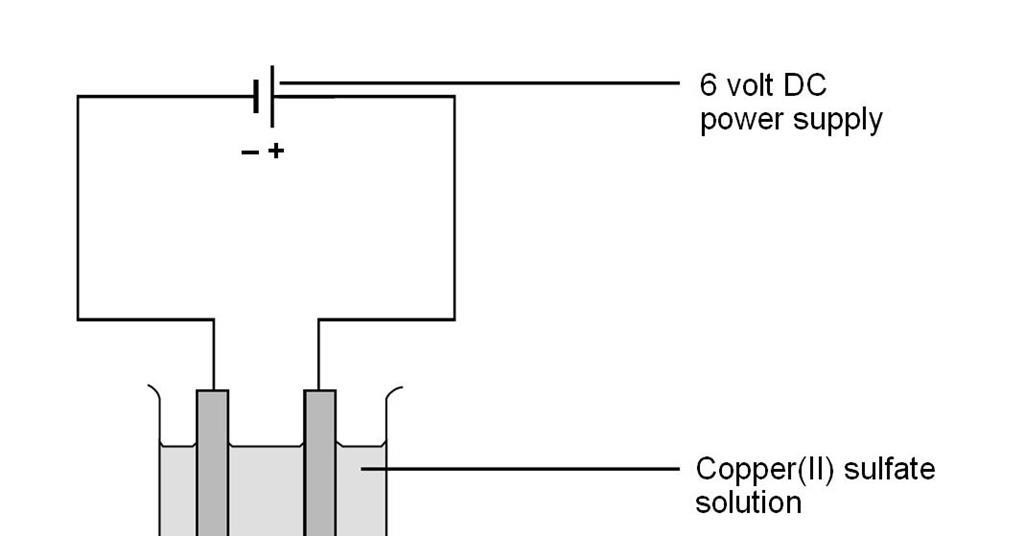
Electrolysis Of Copper Ii Sulfate Solution Experiment Rsc Education

Intercalation Of 1 2 4 Triazole In Methanol Modified Kaolinite Application For Copper Corrosion Inhibition In Concentrated Sodium Chloride Aqueous Solution Sciencedirect

Comments
Post a Comment